Predicting Cancer’s Path
“Precision cancer prevention is a broad, evolving toolkit,” says Chin Hur, MD, MPH, a gastroenterologist at NewYork-Presbyterian/Columbia University Irving Medical Center specializing in early detection. “Image-derived biomarkers and genomics sit alongside multi-cancer early detection blood tests and other liquid biopsies that analyze circulating DNA, RNA, and proteins.”
The near-term role for these blood-based tests, Dr. Hur explains, is to complement—not replace—proven screening, while decision models and implementation research determine who benefits most and how to integrate results into care. “The goal isn’t another standalone test,” says Dr. Hur. “It’s a packaged prediction—radiomics plus the right contextual data—that allows clinicians to intervene earlier and prevent more cancers.”
- Herbert and Florence Irving Professor of Medicine to Honor Dr. Jeffrey Alan Stein, Columbia University Vagelos College of Physicians and Surgeons
- Professor of Epidemiology, Columbia University Mailman School of Public Health
- Director, Healthcare Innovations Research and Evaluation
- Co-Leader, Cancer Population Science Program, Herbert Irving Comprehensive Cancer Center

From Detection to Prediction
The promise of artificial intelligence (AI) in earlier detection is especially compelling. Imaging is central to cancer screening—mammography for breast cancer, low-dose CT for lung cancer—but radiology images contain far more information than the human eye can reliably parse. AI makes that hidden information readable. By extracting measurable features (“imaging biomarkers”) from routine scans and pairing them with clinical and genomic data, researchers can detect patterns that signal who is more likely to develop an aggressive cancer in the future.
At Columbia, a new cross-campus hub—the Center for Innovation in Imaging Biomarkers and Integrated Diagnostics (CIMBID)—is accelerating this shift from detection to prediction. Convening experts in imaging science, machine learning, biomedical informatics, engineering, and clinical oncology, the center’s goal is to turn advanced analytics and AI into integrated diagnostics that are reliable, accessible, and actionable for clinicians and patients.
Leading the effort is Despina Kontos, PhD, professor of radiology and CIMBID’s founding director. A computer scientist by training, Dr. Kontos’s research aims to fundamentally shift how we interpret breast and lung images—using AI to mine scans for observable “signatures” with independent diagnostic, prognostic, and predictive value.
The potential is wide-ranging for cancer care. “Can we take these aspects of AI and have an impact on cancer screening and cancer surveillance?” asks Anil K. Rustgi, MD, director of the Herbert Irving Comprehensive Cancer Center (HICCC), a scientific partner with CIMBID. “Dr. Kontos’s new center, in partnership with the HICCC and others at Columbia, will lead this new frontier, harnessing the power of AI to change the conversation from one of cancer detection to prediction, and ultimately, prevention.”
- Herbert and Florence Irving Professor of Radiological Sciences in Radiology and the Herbert Irving Comprehensive Cancer Center
- Vice-Chair, Artificial Intelligence, Data Science, and Engineering Research, Department of Radiology
- Director, Center for Innovation in Imaging Biomarkers and Integrated Diagnostics (CIMBID)
- Chief Research Information Officer (CRIO), Columbia University Irving Medical Center
- Director of Biomarker Imaging, New York-Presbyterian Hospital
- Member, Cancer Population Science Program, Herbert Irving Comprehensive Cancer Center

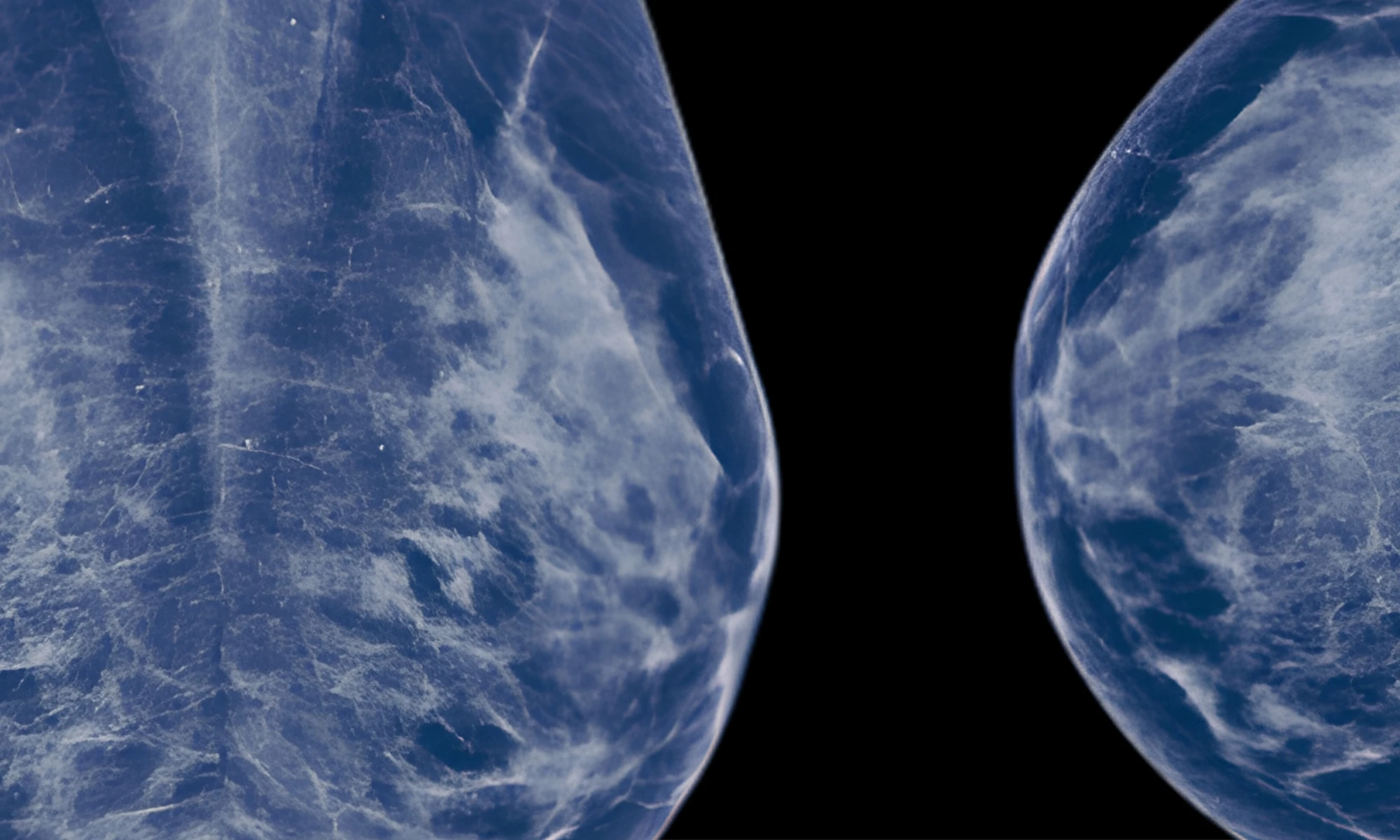
Diving Deeper than Breast Density
Recent work from Dr. Kontos illustrates how using AI in imaging can change the breast cancer playbook. Traditional mammography flags breast density, a known risk factor. But newer algorithms also quantify texture—the micro-patterns created by fat and glandular tissue—that can better predict who will develop cancer down the line, even among individuals with the same density. In a large study analyzing tens of thousands of mammograms, six distinct texture patterns were linked with future breast cancer risk across white and Black women. Embedding that kind of texture-based risk score in routine mammography could personalize screening intervals, escalate surveillance for those at highest risk, and spare lower-risk patients from unnecessary imaging.
Julia McGuinness, MD, a breast oncologist at NewYork-Presbyterian/Columbia University Irving Medical Center, is collaborating with Dr. Kontos to take these concepts into practice. Traditional breast cancer risk models, which are based on factors such as family history, age, and prior biopsy results, can be inaccurate, especially for racial and ethnic minorities where risk can be underestimated. Drs. Kontos and McGuinness are addressing that gap by adding image-derived signals from routine screening mammograms to a widely used risk model. Their AI tool reads subtle mammographic features not visible to the human eye, layering these data with clinical risk factors to potentially provide a more accurate prediction of breast cancer risk. With funding from the Susan G. Komen Foundation, they are broadening this to a larger retrospective study across NewYork-Presbyterian, with the hope of bringing the tool to patients.
“Layering this breast radiomics data with our existing risk models allows us to continue to improve and make breast cancer risk assessment more personalized, more precise,” says Dr. McGuinness.
The same logic extends to lung cancer. Low-dose CT has already reduced mortality in high-risk smokers, but not all “high risk” is the same. Radiomic features—quantitative characteristics of nodules and surrounding tissue—may help distinguish indolent cancer from fast-growing disease and flag patients who would benefit from tighter surveillance or prevention counseling. CIMBID is advancing these multimodal models with partners across Columbia University Irving Medical Center, NewYork-Presbyterian, and Cornell, aligning algorithm development with the everyday questions clinicians and patients ask: What is my risk? What should I do next?
Patient-Centered Precision Prevention
“For patients, precision prevention should feel like clarity and coordination,” says Kontos. “Taking all these disparate data points that make up cancer risk and marrying them into something that you can do something about.” Imagine a 42-year-old whose mammogram looks “routine,” but whose image-derived risk score suggests a higher likelihood of aggressive disease within a few years; her care team recommends a shorter interval to the next screen, a targeted MRI, and a prevention consult. Or consider a long-time smoker whose scan reveals a pattern associated with rapid progression; he receives closer follow-up and support to reduce modifiable risks.
For Dr. McGuinness, the work is personal. Many of her own patients are around her age. Some arrive newly diagnosed with advanced disease despite having no family history. “It reinforces that no one is too young to get breast cancer,” Dr. McGuinness says. “We need to do more to identify those at risk — and intervene to either reduce their risk or detect breast cancer at an earlier, highly treatable stage.”
As precision cancer prevention moves from concept to clinic, HICCC researchers are working to bring clarity to the complex: the right screening approach, at the right interval, for the right patient. The work is integrative both in nature and in practice. “We can’t think about any of this information in isolation,” Kontos says. “We’re using AI to connect the dots, allowing us to anticipate cancer earlier and opening the door to true prevention.”
- Assistant Professor of Medicine, Columbia University Vagelos College of Physicians and Surgeons
- Member, Cancer Population Science Program, Herbert Irving Comprehensive Cancer Center
- Herbert and Florence Irving Professor of Medicine to Honor Dr. Jeffrey Alan Stein, Columbia University Vagelos College of Physicians and Surgeons
- Professor of Epidemiology, Columbia University Mailman School of Public Health
- Director, Healthcare Innovations Research and Evaluation
- Co-Leader, Cancer Population Science Program, Herbert Irving Comprehensive Cancer Center
- Herbert and Florence Irving Professor of Radiological Sciences in Radiology and the Herbert Irving Comprehensive Cancer Center
- Vice-Chair, Artificial Intelligence, Data Science, and Engineering Research, Department of Radiology
- Director, Center for Innovation in Imaging Biomarkers and Integrated Diagnostics (CIMBID)
- Chief Research Information Officer (CRIO), Columbia University Irving Medical Center
- Director of Biomarker Imaging, New York-Presbyterian Hospital
- Member, Cancer Population Science Program, Herbert Irving Comprehensive Cancer Center
- Assistant Professor of Medicine, Columbia University Vagelos College of Physicians and Surgeons
- Member, Cancer Population Science Program, Herbert Irving Comprehensive Cancer Center

